Introduction:
Despite significant improvement in the outcomes of early treatment lines in myeloma, most patients (Pts) invariably relapse. As triple and quadruple regimens are being introduced at the upfront setting, many Pts are either triple-class exposed or triple-class refractory (TCE/TCR) early in their disease course. Although novel immunotherapies are emerging with impressive efficacy among TCE/TCR myeloma Pts, their use is currently limited due to potential toxicities, costs and logistic constraints. We report the outcomes of the IPoD-790 clinical trial (NCT04790474), an all-oral regimen: ixazomib (IXA), pomalidomide (POM) and dexamethasone (DEX) (IPd) combination, in Pts with relapsed refractory multiple myeloma (RRMM) who progressed after bortezomib (BTZ), lenalidomide (LEN) and daratumumab (DARA) therapies.
Methods:
A phase 2, investigator initiated, open-label, single-arm, prospective, multicenter study evaluating the safety, tolerability and efficacy of IPd as a second or third-line combination treatment for TCE RRMM Pts. Pts receive IXA: 3 mg days (d) 1,4,8,11 of 21-d Cycles (Cy) 1-3; 4mg d 1,8,15 of 28-d Cy 4-24. POM 4mg: d 1-14 Cy 1-3; d 1-21 Cy 4-24. DEX 20mg: d 1,2,8,9,15,16 Cy 1-3; d 1,2,8,9,15,16,22,23 Cy 4-24. Treatment continues for 24 cycles, or until disease progression (PD) or unacceptable toxicity (the earliest of the three). After completion of 24 treatment cycles, Pts continue POM DEX as standard of care. The primary endpoint is progression free survival (PFS) according to IMWG criteria.
Results:
Fifty-five (out of the 60 Pts planned) were enrolled; we report data on 46 Pts who had their first dose between March 2021 and April 2023, further data will be presented at the meeting. The median age was 72 (range: 53-88); 18 (39%) were ≥75 years old; 59% were male, ISS was I/II/II for 48% / 25% and 28% respectively; 59% had high risk FISH cytogenetics (t(4:14), t(14:16), +1q21, del17p) with 14% double-hit. Nine percent had extramedullary disease at study enrollment. Exposure rates to BTZ, LEN and DARA were 100%, 98% and 96%, respectively. Forty-one percent had undergone autologous bone marrow transplantation. Refractoriness rates to BTZ, LEN and DARA were 57%, 91% and 65%, respectively. Thirty-seven percent were TCR.
At data cutoff date of 15-Jun-2023, the median follow-up time was 10.4 [95% CI 7.6-13.2] months. Ninety-three percent of the Pts had at least one treatment emergent adverse event (TEAE) and 65% had a TEAE grade 3 or 4. Common (≥ 10%) TEAEs include neutropenia 35% (28% Gr 3 or 4), pneumonia 24% (17% Gr 3 or 4), thrombocytopenia 22% (15% Gr 3 or 4), back pain 22% (2% Gr 3 or 4), asthenia 20% (0% Gr 3 or 4), diarrhea 20% (0% Gr 3 or 4), peripheral edema 20% (2% Gr 3 or 4), COVID19 infection 15% (2% Gr 3 or 4 ), anemia 13% (2% Gr 3 or 4), acute kidney injury (AKI) 13% (7% Gr 3 or 4), rash 13% (0% Gr 3 or 4).
At data cut-off date, 31 Pts (67%) discontinued the study treatment; 20 (43%) due to PD, 7 (15%) due to adverse events (including 3 patients with pneumonia, and 1 patient each with COVID19 infection, campylobacter gastroenteritis, upper gastrointestinal bleeding and AKI) and 4 (9%) died (2 cases of pneumonia, 1 COVID 19 infection and 1 cerebro-vascular accident).
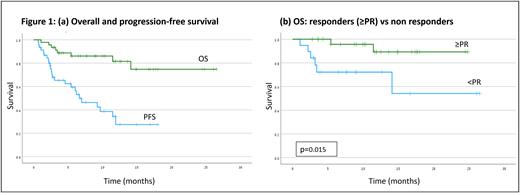
Overall response rate was 59% (27/46); 8 Pts (17%) achieved complete or very good partial response (CR/VGPR), 19 Pts (41%) achieved partial response (PR) and 12 Pts (30%) had stable disease (SD). The median PFS was 6.6 [95% CI 2.9 - 10.3] months (Figure 1a). Median duration of response (DOR) was 10.8 months. Median OS was not reached; 1-year overall survival was 81.6±6.7% (Figure 1a). One-year OS was significantly better for responders (89.3±7.3%) than non-responders (72.2±10.7%), p=0.015 ( figure 1b).
Conclusions:
IPd combination with a twice-weekly ixazomib induction provided an all-oral safe and efficacious therapy in triple-exposed RRMM Pts, most of whom were refractory to both DARA and LEN. Response rates and PFS are encouraging, compared to parenteral PI and IMiD combination regimens in TCE/TCR MM, with a favorable safety profile and convenience, especially among an elderly and frail population with relapsed myeloma.
Disclosures
Cohen:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ganzel:Investigator initiated study, supported by JAZZ company.: Research Funding. Mittelman:FibroGen: Other: participated in clinical trials; Janssen: Research Funding; Medison/Amgen: Research Funding; AbbVie: Other: participated in clinical trials, Research Funding; Geron: Other: participated in clinical trials; Astellas: Other: advisory boards; Silence: Other: advisory boards; Onconova: Other: advisory boards; Media Digital: Speakers Bureau; Celgene/BMS: Other: participated in clinical trials, Research Funding, Speakers Bureau; Takeda: Other: participated in clinical trials, advisory boards; Novartis: Other: participated in clinical trials, advisory boards, Research Funding, Speakers Bureau; Roche: Research Funding; MDS HUB: Consultancy; Gilead: Consultancy, Research Funding. Avivi Mazza:AbbVie: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal